Group leader: Prof. dr. Pieter Rein ten Wolde
About us
Biochemical networks are the central processing units of the living cell. They allow the cell to sense, transmit, amplify, multiplex, and integrate signals, and even enable it to predict future signals. In our group we use ideas from statistical mechanics and combine analytical theory with innovative computational techniques to elucidate their design principles.
Figure Snapshot of a simulation of a signal transduction pathway (MAPK)
The Biochemical Network group develops and applies new numerical techniques that make it possible to simulate biochemical networks at the particle level in time and space
Recent highlight
Bacteria opt for the best price-to-quality ratio to predict the future
Predicting the future can be a matter of life or death. Just think, every time you cross the street, you predict whether this is possible without being run over. Experiments show that even single-celled organisms such as bacteria can predict the future. The better bacteria can predict changes in their environment, the greater their chances of survival. AMOLF researchers from the theory group of Pieter Rein ten Wolde discovered that E. coli bacteria collect the information with the best price-to-quality ratio to predict the future. The researchers publish the results on October 4th in the journal PNAS.
Any system, be it a human, bacterium, or robot, must predict the future based on information from the past. That first of all requires this information to be stored. Whereas we do that in our brain, bacteria do it with the help of proteins and energy in the cell, a so-called biochemical network. Ultimately, how well a system can predict the future is always limited by the amount and quality of information from the past that it has stored.
The price-to-quality ratio of information
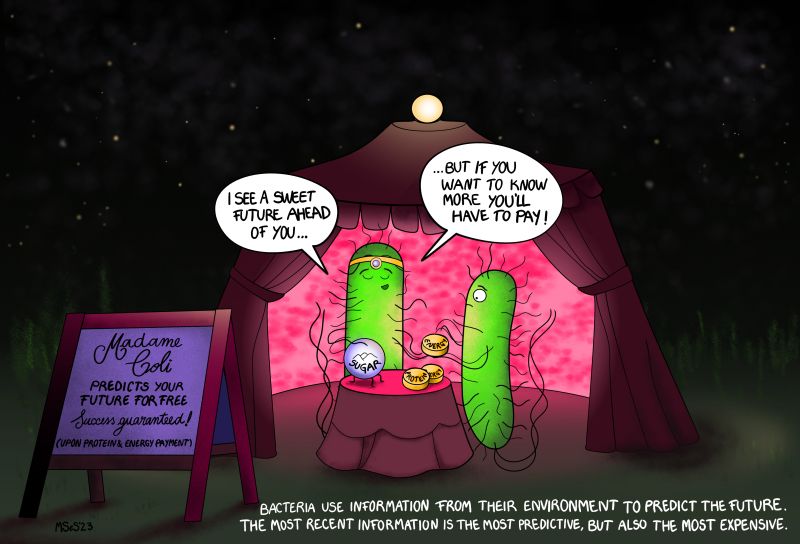
The work of the researchers revealed that E. coli does not collect the most predictive information. This surprised them because if better predictions offer a greater chance of survival, then why would bacteria not use the most reliable information for making these predictions? Further analysis revealed that E. coli does indeed employ a different approach, namely collecting information with the best price-to-quality ratio. This choice is underpinned by an interesting principle.
The most recent information is the most valuable: if you know where a car is now, you have a greater chance of crossing the street safely than if you know where that car was ten seconds ago. However, obtaining recent information is also costly. For that reason, if you need to collect as much predictive information as possible given a fixed budget, it can pay off to store a greater amount of less reliable but cheaper information. The price-to-quality ratio of this information is better.
Given the available budget in terms of proteins and energy, E. coli therefore makes the best possible prediction. This shows that the price of information plays an important role for simple bacteria such as E. coli.
The identified principle, namely that systems have to make a trade-off between the predictive value of information and the price of the information, extends well beyond just E. coli. The developed theory does not only improve our understanding of current biological systems but can, for example, also be used to design efficient robots with minimal energy consumption.
Reference
Age J. Tjalma, Vahe Galstyan, Jeroen Goedhart, Lotte Slim, Nils B. Becker, Pieter Rein ten Wolde, Trade-offs between cost and information in cellular prediction, PNAS, 120 (41) e2303078120, October 4 (2023).

