How do stem cells choose their identity?
AMOLF researchers discovered that stem cells first specialize into a functional cell and then move to the proper location – rather than the other way around.
Researchers at AMOLF and the Hubrecht institute revealed a new model for how stem cells specialize into a functional cell. They found that their position in the organ is not as important as current models claim. Stem cells rather choose their identity first and only then move to the appropriate position. The discoveries were made using intestinal organoids and the new TypeTracker technique, which can now be used to understand other organs at the cellular level, and the effects of mutations and medications. The findings are published on August 18th in the scientific journal Science Advances.
Our intestines contain different types of cells, each of which has a specific task. Just like in many other places in our body, the cells in the intestines are constantly renewed: stem cells develop into specialized cells that perform a function, for example, to secrete substances that protect the intestine or to absorb nutrients from food.
“From previous research we know that stem cells reside in the valleys of the intestinal wall (the ‘crypts’), while most specialized and functional cells are located at the top of the mountains (the ‘villi’)”, say Sander Tans and Jeroen van Zon, who directed the research jointly at AMOLF. “The cells in the intestinal wall are renewed about every week, using the stem cells in the crypts that grow, divide and migrate to the villi. We used to think that by moving upwards to the villus, the stem cells are instructed to become a functional cell. This has been a very appealing model, as it naturally explains how these functional cells are positioned at the right location. However, our data shows a different picture.”
Organoid
This data was obtained using organoids: mini-organs that mimic the original organ so realistically that scientists can use them to unravel its functioning or to test medicines. PhD student Xuan Zheng developed the new TypeTracker technique to study the specialization of stem cells. “I first take a 3D movie of a growing organoid for about sixty hours”, says Zheng. “Next, I analyze these recordings using Artificial Intelligence, which gives coordinates of all cells as they move and divide, and hence also the cellular family trees.”
The researchers added a step to this technique that led to surprising insights. Zheng: “The identity of the cells is determined by certain proteins. But one cannot visualize all relevant proteins during the growth process. So instead, after taking the movie I used fluorescent and dyed antibodies that specifically bind these proteins, to visualize the identity of the cells. I realized that because of the structure of family trees, I can then also show when cell identities changed in the past. It is like a family tree for humans: if one part of the family has a certain disease but the other doesn’t, you can trace the family tree back in time to determine when that mutation arose.”
This new type of data showed that stem cells adopted their functional identity much earlier than previously thought. They did so when still deep inside the crypt, before migrating towards the villus region that was thought to provide the trigger to start the specialization process.
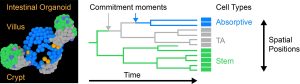
Commit-then-sort model
Based on these cellular family tree experiments, Zheng formulated a new model for how intestinal stem cells specialize, which the researchers call the ‘commit-then-sort’ model. “We now know where and when intestinal stem cells start to specialize. This has implications for all kinds of other research”, says Zheng. “Various medical conditions are thought to be caused by an imbalance between cell types. For instance those that secrete hormones, which has been linked to intestinal bowel syndrome (IBS), the sensation of fullness, but also the so-called gut-brain axis. Understanding how cells choose their identity is key to uncovering the regulation of this balance, and to controlling it through medical interventions. Furthermore, if we want to better understand which molecular signals underpin the fate choices, we need to look into the earlier stages, when cells still have a strong stem identity and other known molecular signals, such as the WNT pathway that plays a role in cell specialization, are still high.”
The equipment and procedure for the TypeTracker method is relatively simple. Therefore, it is also promising for all kinds of other research on organoids. “Cell identity is central to all organ functions, and was previously only known in static pictures. This method allows one to look at the dynamics at the cellular level. One can for instance investigate whether the same commit-then-sort principle holds for other organs with a completely different three-dimensional structure, such as breast tissue that consists of channels,” says Zheng. “The beauty of organoids is that you can follow the growth program at the cellular level under the microscope, and how it changes due to genetic mutations, medicines or harmful substances. Ultimately, we hope to unravel the molecular triggers that determine how and when stem cells specialize.”
Reference
Xuan Zheng, Max A. Betjes, Pascal Ender, Yvonne J. Goos, Guizela Huelsz-Prince, Hans Clevers, Jeroen S. van Zon, Sander J. Tans, Organoid cell fate dynamics in space and time, Science Advances, August 18 (2023), DOI: 10.1126/sciadv.add6480


