Scientists discover how cells envelop bacteria
The protein GBP1 is a vital component of our body’s natural defence against pathogens. This substance fights against bacteria and parasites by enveloping them in a protein coat, but how the substance manages to do this has remained unknown until now. Researchers from Delft University of Technology and the AMOLF have now unravelled how this protein operates. This new knowledge, published in Nature Structural & Molecular Biology, could aid in the development of medications and therapies for individuals with weakened immune systems.
So-called Guanylate Binding Proteins (GBPs) play a crucial role in our innate immune system, explains biophysicist Arjen Jakobi: “GBPs form the first line of defence against various infectious diseases caused by bacteria and parasites. Examples of such diseases include dysentery, typhoid fever caused by Salmonella bacteria, and tuberculosis. The protein also plays a significant role in the sexually transmitted infection chlamydia as well as in toxoplasmosis, which is particularly dangerous during pregnancy and for unborn children.”
Coat around bacteria
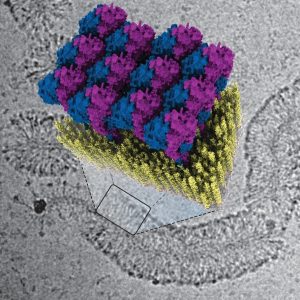
In their publication, Jakobi and his colleagues describe for the first time how the innate immune system fights against bacteria using GBP1 proteins. “The protein surrounds bacteria by forming a sort of coat around them,” explains Tanja Kuhm, PhD candidate in Jakobi’s research group and the lead author of the article. “By pulling this coat tighter, it breaks the membrane of the bacteria—the protective layer surrounding the intruder—after which immune cells can clear the infection.”
Deciphering the defence strategy
To decode the defence strategy of GBPs, the researchers examined how GBP1 proteins bind to bacterial membranes using a cryogenic electron microscope. This allowed them to see the process in great detail down to the scale of molecules. Jakobi: “We were able to obtain a detailed three-dimensional image of how the protein coat forms. Together with biophysical experiments conducted in Sander Tans’ research group at AMOLF, which enabled us to manipulate the system precisely, we succeeded in deciphering the mechanism of the antibacterial function.”
By using an optical tweezer to hold a membrane vesicle under the microscope, PhD student Luca Gross at AMOLF was able to directly investigate the effect of GBP1. Gross: ‘In the experiments, I saw that the membrane vesicle was cut into small pieces after adding GBPI. It was fascinating to see how these proteins can autonomously exert force on the membrane to break it down’.
Medications
According to Jakobi, this research helps us understand better how our body is capable of combating bacterial infections. “If we can grasp this well, and we can specifically activate or deactivate the involved proteins through medication, it may offer opportunities to speed up getting rid of certain infections.”
Reference
Tanja Kuhm, Clémence Taisne, Cecilia de Agrela Pinto, Luca Gross, Evdokia A. Giannopoulou, Stefan T. Huber, Els Pardon, Jan Steyaert, Sander J. Tans & Arjen J. Jakobi, Structural basis of antimicrobial membrane coat assembly by human GBP1, Nature Structural & Molecular Biology, 11 October (2024).
Contact
Do you have questions related to this research? Then please contact Sander Tans, email: S.Tans@amolf.nl.


