Superresolution imaging in living cells
The use of fluorescent proteins such as GFP allows for in vivo labeling of native proteins at high specificity to achieve high-contrast imaging of their spatial organization within a cellular context. Yet information attainable by traditional fluorescence microscopy is fundamentally limited by the so-called diffraction limit of spatial resolution—even with microscope objectives of highest performance, one can only achieve resolution of roughly half the wavelength of light (~250 nm for green fluorescence). This means that molecules separated by less than this distance cannot be distinguished from one another, placing strong constraints on practical applications that are particularly relevant for the bacterial cell (which measures only several-fold larger than this diffraction-limited length scale). There are at least two ways in which the diffraction limit can hinder practical applications. For situations in which the spatial arrangement of molecules is of interest, the diffraction limit effectively blurs the image of the arrangement, thereby obscuring relevant details regarding shape and/or size of cellular structures. For situations in which the spatial dynamics of molecules are of interest, the diffraction limit hinders reliable tracking of movement, as the blurred image of molecules or molecular assemblies often overlap with one another.
In our group, we use two imaging methods that utilize photo convertible fluorescent proteins to overcome each of these difficulties. Photoactivation localization microscopy (PALM), originally developed by Betzig et al.1, allows one to image the spatial organization of proteins with increased spatial resolution within fixed cells. Localized photoactivation single-particle tracking (LPA-SPT), developed in our group2,3, allows one to study the movement of single molecules as well as clusters of molecules (e.g. protein signaling complexes) within the crowded environment of live cells.
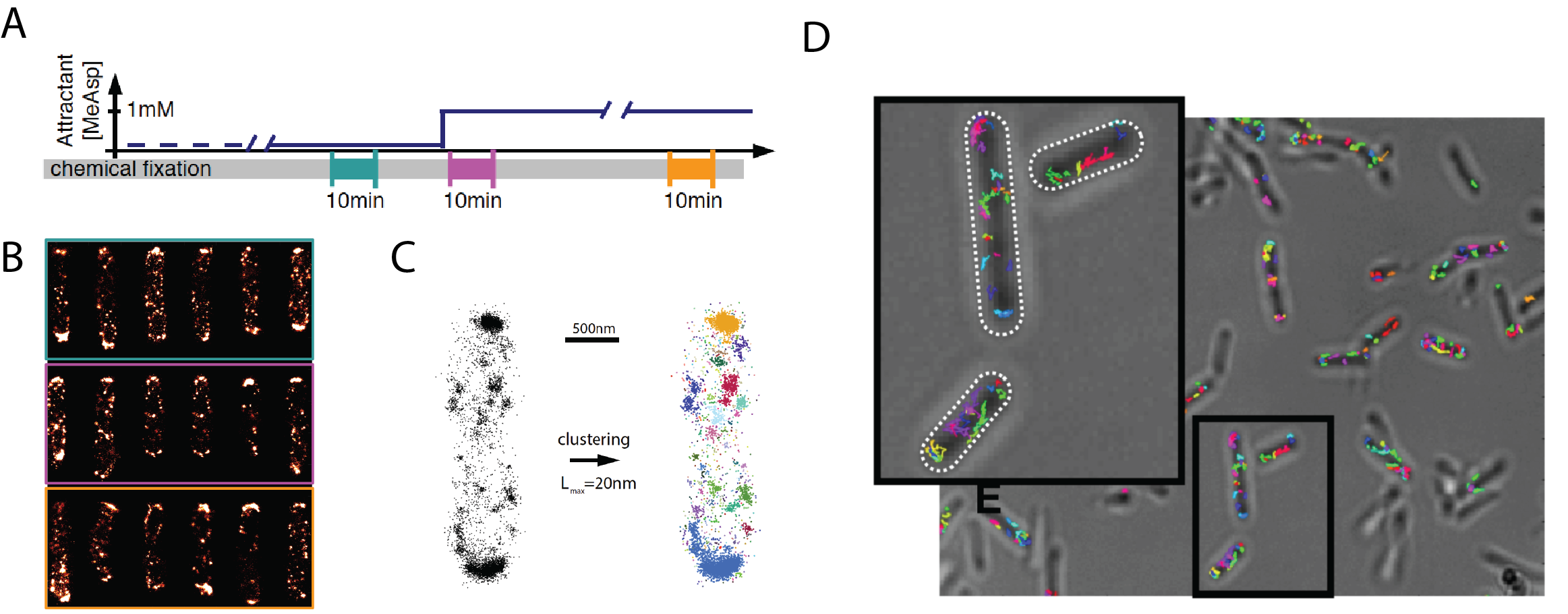
Fig. 1. Revealing dynamical changes in nanoscale spatial organization in live bacteria. (A) To assess whether chemoreceptor clusters, which amplify and integrate sensory signals in bacteria, change their size and mobility depending on stimulus history, we apply both PALM and LPA-SPT imaging at different times before and after stimulation by ligand stimuli. (B) Superresolution PALM images of of the chemoreceptor Tar (labeled with mEOS2) in cells fixed at the times indicated in (A). (C) Clustering analysis on the localization coordinates allows detailed analysis of the nanoscale spatial organization under various signaling states. (D) LPA-SPT provides dynamic information about the mobility of single molecules and clusters of chemoreceptors under sensory stimulation and adaptation.
References
- Betzig, E., Patterson, G.H., Sougrat, R., Lindwasser, O.W., Olenych, S., Bonifacino, J.S., Davidson, M.W., Lippincott-Schwartz, J. and Hess, H.F. (2006). Imaging intracellular fluorescent proteins at nanometer resolution. Science, 313:1642-1645.
- Solari, J., Anquez, F., Scherer, K. & Shimizu, T.S. (2018). Bacterial chemoreceptor imaging at high spatio-temporal resolution using photoconvertible fluorescent proteins. Meth Mol Biol 1729:203-231.
- Koler, M., Peretz, E., Aditya, C., Shimizu, T.S. & Vaknin, A. (2018). Long-term positioning and polar preference of chemoreceptor clusters in E. coli, Nature Commun:9, 4444.