Proteins absorb water before unfolding
Proteins perform specific biological functions for which they strongly depend on their three-dimensional structure that results from the folding of the polypeptide chain. The mechanisms by which proteins fold and unfold are still not fully understood.
A crucial factor for protein folding is the interaction between a protein and the water molecules that surround it. Scientists in the group of Huib Bakker studied this interaction and observed for a range of proteins that they absorb water before unfolding.
The scientists, Huib Bakker and Carien Groot, measured the reorientation dynamics of water in protein solutions using polarization-resolved femtosecond infrared spectroscopy. Some of the water molecules move around at a much slower pace because of their interaction with the protein surface. By monitoring those slowly moving water molecules the researchers can directly probe the amount of protein surface that is exposed to water.
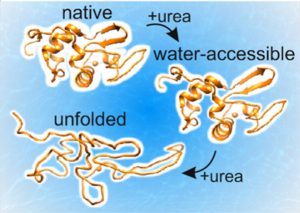
Being able to detect the exposure of the protein surface to water molecules, the scientists were able to take the next step. They studied the folding process using urea, which is a compound known for its capacity to unfold proteins. When the urea concentration is low the water exposed surface of the proteins increases by almost 50%, indicating that the proteins absorb water. However, the secondary structure (α-helices, β-sheets) is still intact. This finding indicates that protein unfolding starts with the protein becoming less tight, absorbing water molecules.
The full study can be read in the Journal of Physical Chemistry Letters:
http://pubs.acs.org/doi/abs/10.1021/acs.jpclett.6b00708
Schematic picture of protein unfolding, illustrating the transition from native protein (top) to protein at low urea concentration (middle), to unfolded protein (bottom). At low urea concentrations, the protein is more accessible to water while the secondary structure is still intact.